Publications
FIP’s publications in this area range from the FIP-WHO Good Pharmacy Practice guidelines (2011) to advocacy tools, global data, guidelines and practice support tools. They include a members-only advocacy toolkit to support member organisations’ advocacy for the implementation of pharmacy-based vaccination, a collection of evidence and guidelines for the development of vaccination services, survey reports on the evolving roles of pharmacy in vaccination, and a regulatory self-evaluation assessment tool for advancing pharmacy services in this area.
Publications are presented in chronological order, from more recent to older. Please scroll down inside the grey area or using the scroll bar on its side to find more FIP publications.
Leveraging pharmacy to deliver life-course vaccination: An FIP global intelligence report – Executive summary (2024)
As more countries authorize pharmacists to deliver vaccines, FIP remains committed to supporting pharmacists in this vital role and advocating for the widespread use of pharmacists in ensuring vaccination coverage and improving the health care system. A comprehensive overview of pharmacist-led vaccination services around the world is presented in this executive summary, which is open access.
Leveraging pharmacy to deliver life-course vaccination: An FIP global intelligence report (2024)
Recent years have seen pharmacists’ roles in immunization evolve significantly, with increased access to administer vaccines across regions. Based on a cumulative dataset of 120 countries, this report evaluates many aspects of pharmacist-led vaccination. These include advocacy activities, regulatory frameworks, vaccination administration and prescribing, training, certification, access to vaccination records, remuneration models and identifying barriers to extending these services. This study forms part of the FIP Vaccination Surveillance Project, an ongoing initiative aimed at continuously monitoring and assessing the development of pharmacists’ roles in immunization globally.
Advancing pharmacy practices in vaccination: Preparing for winter. Report from a FIP insight board (2024)
This report, resulting from an insight board that FIP hosted in October 2023, aims to shed light on the multifaceted approach undertaken by pharmacies to meet the increased demand for immunisations during winter, considering factors such as vaccine availability, staff training, public awareness campaigns and collaborative efforts with healthcare stakeholders. At the insight board, experts in the field of immunisation discussed vaccination and preparing for winter and shared valuable insights for pharmacists, healthcare professionals, policymakers and the general public to better understand and appreciate the vital role of pharmacies in increasing uptake of different vaccines, especially during winter periods.
Advancing pharmacy practices in vaccination: Reaching at-risk and vulnerable groups. Report from a FIP insight board (2024)
During an insight board hosted by FIP in October 2023, experts in pharmacy and immunisation joined to discuss ways of improving vaccination for at-risk and vulnerable population groups. This report provides a summary of the insight board discussion as well as the key insights that were shared.
Advancing pharmacy practices in vaccination: Unlocking vaccine confidence. Report from a FIP insight board (2024)
FIP, being the leader of pharmacy globally, continues to expand its presence within pharmacy and pharmaceutical sciences, and to influence policymaking, education and science institutions to become better. As part of its efforts on vaccination, FIP hosted an insight board in October 2023 exploring the factors that affect vaccine confidence and public trust in vaccines as well as the strategies that optimise vaccine acceptance.
Vaccination: Benefits beyond specific diseases prevention. Summaries from the FIP digital events programme (2023)
This report provides a summary of the outcomes of two of the events that mostly took part as panel discussions (Part 3 and Part 4), highlighting key insights from panel discussions that showcased the burden of diseases and the risks and costs of not widening access to vaccination as well as the key role that pharmacists can play in life course immunisation.

Pharmacy-based vaccination: Recent developments, success stories and implementation challenges (2023)
This report provides an update in terms of qualitative insights on recent developments and success stories of pharmacy-based vaccination in several countries, as well as an overview of some of the challenges that some countries are still facing to introduce this valuable professional service by pharmacists. The report also aims to uphold FIP’s global and regional advocacy of vaccination-related roles by pharmacists, and to provide FIP member organisations with examples that can support their own process and advocacy. The report includes a compilation of 17 case studies and country updates by FIP member organisations from countries with remarkable recent developments in this area, followed by the report from an insight board (focus group) discussion with countries that are at different stages of introducing pharmacy-based vaccination or that are facing specific challenges to achieve this goal.
Statement of policy on the role of pharmacy in life-course vaccination
Read Publication

Supporting life-course immunisation through pharmacy-based vaccination: enabling equity, access and sustainability. A toolkit for pharmacists (2023)
Health equity is only achieved when there are no differences in the quality of and access to health care among all groups and at all ages in a society. Whether to an infant, a child, a young adult, or an older adult, the quality of health care should not change. Similarly, access to care should not vary throughout an individual’s life. Because inequities are cumulative, their impacts are not confined to one stage of an individual’s life but rather remain and affect a person’s
life years afterwards. In addition, the impacts are not restricted to a particular individual, but can affect the lives of those around. Vaccine inequities, too, especially among people of different ages, have a cumulative effect. Inequitable access to vaccines not only leaves people at risk of contracting and spreading deadly viruses but also leads to more virus variants emerging, affecting a whole population, even those vaccinated.

FIP commitment to leveraging pharmacists to build vaccine confidence and address vaccine hesitancy and complacency (2022)
Vaccine hesitancy – concerns related to vaccination or outright refusal to receive vaccines despite availability – is a major threat to global health and an important barrier to the success of vaccination strategies worldwidei. Barriers such as misinformation and distrust in vaccines can compromise not only the health of individuals but also public health as a whole.
The International Pharmaceutical Federation (FIP), which brings together 146 organisations of pharmacists, pharmaceutical scientists and pharmaceutical educators, and represents over four million pharmacists from around the world, believes it is essential that pharmacy and other civil society organisations join forces and outline synergistic and complementary advocacy actions for broader access to and convenience of vaccination services through a diversity of providers and pathways, and to address vaccine hesitancy from multiple perspectives.
The commitment is available in the following languages:
Vaccination of special-risk groups: A toolkit for pharmacists (2022)
Pharmacists are one of the most easily accessible healthcare professionals, and they are increasingly engaged in primary healthcare strategies, including disease prevention. Pharmacists are key healthcare professionals to support specific population groups who are more vulnerable to severe forms of vaccine-preventable diseases and have a higher risk of hospitalisation, loss of functional ability and even death. These special-risk groups must be the target of proactive and systematic actions by healthcare professionals to ensure they are vaccinated against all diseases that may impact their health in a severe and largely preventable way.
Videos: Vaccines and special-risk population groups
While vaccines are recommended for almost everyone, there are specific groups of people who may be particularly vulnerable to severe forms of vaccine-preventable diseases. Their health conditions may increase the risk of developing complications requiring hospitalisation, developing secondary illnesses or even dying. Therefore, it is imperative to actively promote vaccination to those groups to ensure that they receive all the vaccines that are suitable for their age and clinical situation. Pharmacists can play an important role in promoting and advocating vaccination to these groups by leveraging their accessibility, expertise and trust by the population. Their frequent interactions with the public provide valuable opportunities to raise awareness of the importance of vaccination.
Optimising vaccination through coadministration of influenza and COVID-19 vaccines: Guidance for pharmacists (2022)
This new guidance for pharmacists explores how vaccination strategies for two important respiratory conditions — COVID-19 and influenza — can and should be considered together and combined in an effective and impactful way to improve uptake rates for each vaccine.

The FIP vaccination reference guide. Knowledge and skills to support professional development and inform pharmacy education in vaccination (2022)
Pharmacists in many parts of the world play key roles in public health, including vaccination-related services. As
established advocates, educators and qualified providers of vaccines, pharmacists have a significant role in promoting
and supporting the uptake and monitoring of vaccination. Their roles include raising awareness of the benefits of
vaccination and improving immunisation coverage. To successfully deliver these important roles, pharmacists need to
obtain the required knowledge and skills in the area of vaccination.
“FIP vaccination reference guide: Knowledge and skills in pharmacy education and professional development” is
developed by FIP for educators and academic institutions, practitioners and professional organisations, and
policymakers. The reference guide outlines the knowledge and skills that should be acquired by students during their
undergraduate pharmacy education, and expands into the knowledge and skills that should be obtained by pharmacists
through professional development and training.

Advocating expansion of the pharmacist’s role in immunisation: A focus on diphtheria-tetanus-pertussis booster, COVID-19 and meningitis vaccinations (2022)
Pharmacists are contributing to expanded immunisation coverage globally, thereby reducing illness and deaths from vaccine-preventable diseases. The report provides intelligence on the current status of these services in 36 countries that have regulated pharmacy-based vaccination services to identify pharmacists’ current role in DTP booster vaccination, COVID vaccination and meningococcal meningitis vaccination. The new data shows that their role has expanded since the publication of a previous FIP report in 2020. This increase has been driven in part by the need for urgent and mass vaccination as a result of the COVID-19 pandemic, the report authors say. In addition, vaccination authority policy development, stakeholder engagement and acceptance of pharmacists’ role, logistics development, and education and training were highlighted as being necessary if pharmacists’ role in vaccination services is to be leveraged further.
Crear confianza en las vacunas y comunicar su valor: Guia para farmacéuticos (2021)
Esta publicación identifica motivos comunes de preocupación o vacilación relacionados con la seguridad y eficacia de las vacunas y propone enfoques efectivos para abordarlos de diversas maneras a través de campañas dirigidas por farmacias y mediante interacciones con pacientes individuales.
Construir a confiança nas vacinas e comunicar o seu valor. Um manual para farmacêuticos. (2021)
Esta publicação identifica razões comuns de preocupação ou hesitação relacionadas com a segurança e eficácia das vacinas e propõe abordagens eficazes para as abordar de diversas formas através de campanhas lideradas pelas farmácias e através de interacções com pacientes individuais.
Building vaccine confidence and communicating vaccine value: A toolkit for pharmacists (2021)
This publication identifies common reasons for concern or hesitancy related to vaccine safety and efficacy and proposes effective approaches to address them in a variety of ways through pharmacy-led campaigns and through interactions with individual patients.
FIP Vaccination Handbook for Pharmacists (2021)
With this publication, FIP aims to support individual pharmacists with understanding how they can contribute to improving vaccination coverage through services ranging from patient education and advice, to logistical roles and the administration of vaccines. This handbook provides guidance on the practical implementation of these services and includes guidelines on the procedures, safety aspects, common risk points and frequently asked questions about vaccines and their administration.
Programme de vaccination contre la pandèmie en pharmacie: Un outil d’auto-évaluation des politiques (2021)
Cet outil d’auto-évaluation permettra d’identifier les points forts et les domaines à améliorer afin d’informer les efforts de planification pandémique avant les vagues actuelles et futures de COVID-19. Il s’agit d’un outil destiné à aider les ministères de la santé et les organismes de réglementation nationaux à évaluer la préparation à la pandémie et l’état de la législation et de la réglementation dans leur pays afin de faciliter la vaccination et le dépistage de masse et d’améliorer les soins aux patients par le biais des pharmaciens et des pharmacies communautaires.
FIP call to action to expand the role of community pharmacies in vaccination, including against COVID-19 and future pandemic (2020)
This statement highlights the role of pharmacists in vaccination and in the promotion of vaccination coverage around the world.
An overview of pharmacy’s impact on immunisation coverage. A global survey (2020)
This report contains the findings of FIP’s latest survey (2020) of pharmacy’s impact on immunisation coverage. It evaluates different aspects of pharmacist-led immunisation, including advocacy and awareness activities, regulatory frameworks, vaccine administration, reimbursement models, training and certification, vaccination records, and limitations and barriers to the expansion of pharmacy practice to include vaccine administration. A total of 99 countries and territories participated, making this the most comprehensive report published on this subject to date.
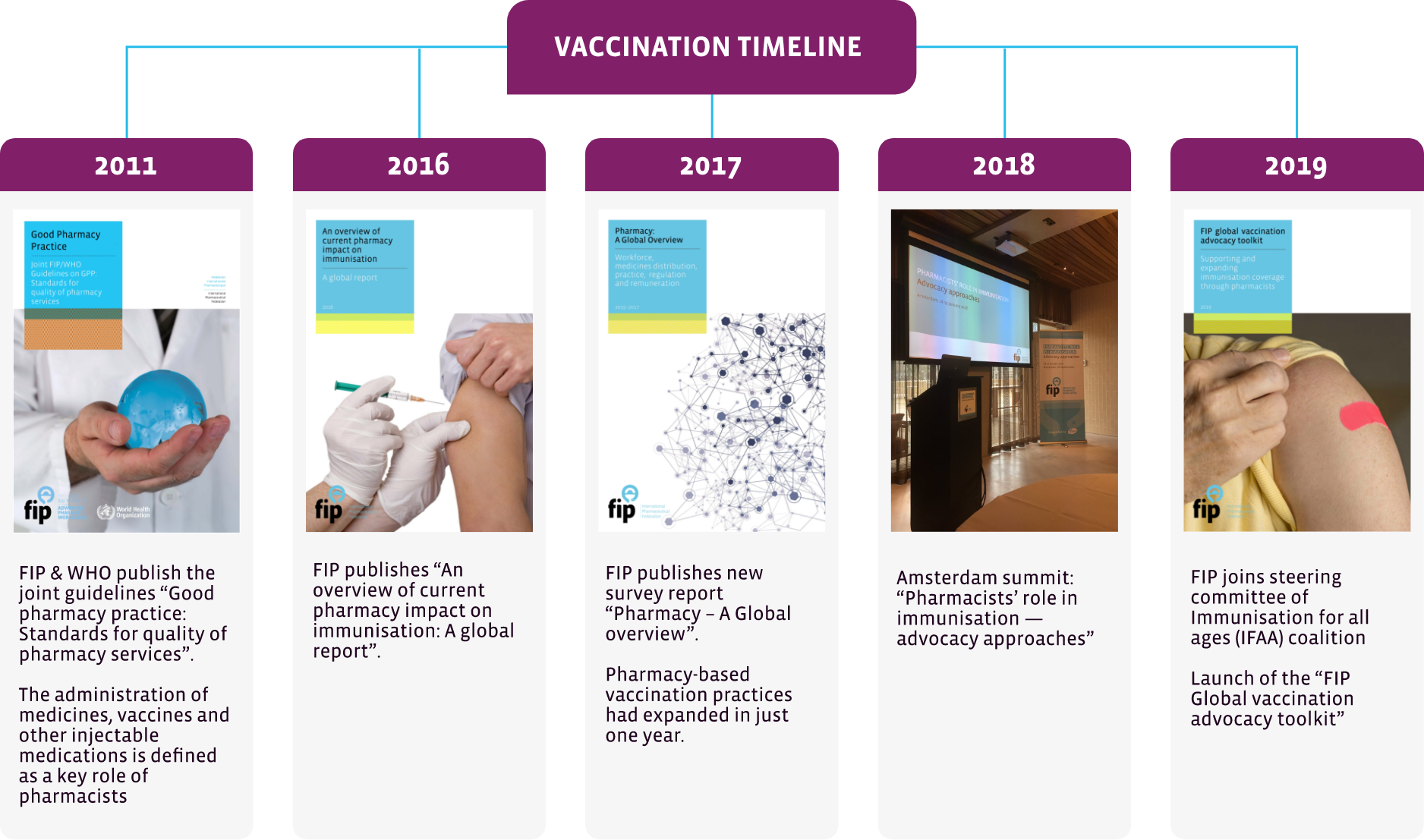
An overview of current pharmacy impact on immunisation. A global report (2016)
This publication reports the findings of the first FIP survey on the roles of pharmacists in vaccination, conducted in 2016.
Good Pharmacy Practice – Joint FIP/WHO Guidelines on GPP Standards for quality of pharmacy services (2011)
This document describes the key roles, functions and activities of pharmacists, including the administration of vaccines.
Events/Webinars
FIP organises digital events and webinars regularly to highlight different topics related to vaccination, share best practices and innovations and advocate for the role that pharmacy can play in improving access to vaccination services.
You may access the recordings of all past events through the links provided below. Events are presented in chronological order, from more recent to older. Please scroll down inside the grey area or using the scroll bar on its side to find more FIP events. You may also want to check the upcoming digital events at www.events.fip.org.
Reaching at-risk and vulnerable groups: Pharmacy’s role in inclusive vaccination
November 14, 2023
FIP is organizing a series of three webinars centred on critical aspects of vaccination. These webinars aim to provide insights into three key topics to advance pharmacy-based vaccination services:
1. The first event is dedicated to addressing vaccine confidence and aims to explore the factors that influence public trust in vaccines and discuss strategies to bolster vaccine acceptance.
2. The second event focuses on preparing for vaccination campaigns during the winter season. This includes discussions on adapting vaccination strategies for seasonal challenges and ensuring the availability of vaccines during this crucial period.
3. The third event focuses on at-risk or vulnerable groups and examines the importance of reaching and safeguarding these populations and explores ways in which pharmacies can play a crucial role in their vaccination.
Watch video
Preparing for winter: Pharmacy-based vaccination strategies
November 9, 2023
One of the key objectives of this webinar series is to harness the insights gained from the panel discussions to inform the development of global guidelines for pharmacy practices related to vaccination. These guidelines will serve as a valuable resource for the international pharmacy community, contributing to more effective and inclusive vaccination efforts worldwide.
Watch video
Unlocking Vaccine Confidence: A Pharmacy Perspective
November 2, 2023
One of the key objectives of this webinar series is to harness the insights gained from the panel discussions to inform the development of global guidelines for pharmacy practices related to vaccination. These guidelines will serve as a valuable resource for the international pharmacy community, contributing to more effective and inclusive vaccination efforts worldwide.
Watch video
Vaccination: Benefits beyond specific disease prevention – Part 4
October 23, 2023
This is the 4th and final episode in the FIP series ‘Vaccination: Benefits beyond specific disease prevention’ which aimed to highlight the extensive advantages of vaccination beyond merely guarding against specific pathogens in older adults.
Parts 1 and 2 focused on the evidence base of indirect benefits of vaccination in areas such as cardiovascular health, diabetes and arthritis in older adults and prolonged immune responses that contribute to sustained health. They also underscored the indispensable role of pharmacists in promoting healthy aging through vaccination.
Vaccination: Benefits beyond specific disease prevention – Part 3
September 13, 2023
Building on the Part 2 of the series, Part 3 will focus on the burden of diseases and the risks and costs of not widening access to vaccination. FIP resources will be revisited as well as the process of setting up vaccination at a pharmacy: pre-vaccination, vaccination, post-vaccination and in-depth case studies. The event will take the format of a brief presentations followed by a panel discussion in which experts will join in to share their knowledge and expertise.
Watch video
Vaccination: Benefits beyond specific disease prevention – Part 2
August 31, 2023
We all know that the primary goal of vaccination is the prevention of pathogen-specific infection. But what about the indirect benefits of vaccines which drive longer-term immune responses that promote long-term health? This webinar – Part 2 – will share contributions, activities, tools that pharmacists lead and utilise to promote healthy aging in the practice setting. The webinar will highlight the varied but crucial role of pharmacists in vaccination, particularly for older adults, and supporting patients’ quality of life.
Watch video
Vaccination: Benefits beyond specific disease prevention – Part 1
August 21, 2023
We all know that the primary goal of vaccination is the prevention of pathogen-specific infection. But what about the indirect benefits of vaccines which drive longer-term immune responses that promote long-term health? This webinar – Part 1 – will delve into technical and evidence-based insights on positive downstream effects of vaccines in older adults including that on cardiovascular events and diseases, diabetes or arthritis
Watch video
Enabling life-course immunisation through pharmacy-based vaccination: Launch of a new FIP Pandemic Preparedness report
August 16, 2023
Following the pandemic, how to continue to lead the profession globally and support our members during those uncertain times became a core priority for FIP in 2023. As we move on from responding directly to COVID-19 to responding to the aftermath and to readying ourselves for future pandemics and health emergencies, FIP sought to derive key lessons, recommendations and considerations for the future in another report, “Pandemic Preparedness, Response and Recovery: Lessons Learnt for Global Pharmacy”.
Watch video
Enabling life-course immunisation through pharmacy-based vaccination: Launch of a new FIP policy toolkit
July 26, 2023
FIP is committed to supporting countries to develop policies that enable pharmacies and pharmacists to deliver integrated vaccination services throughout the life-course and as part of wider national immunisation policies. This event is delivered as part of a new FIP programme supported by Pfizer, which aims to provide our members with approaches and tools for tackling key policy enablers that determine equity, access and sustainability of pharmacy-based life-course immunisation.
The success of pharmacy-based vaccination depends on various policy factors, one of which is access to data and vaccination records. FIP data shows that pharmacists’ access to patient vaccination records vary widely across countries and regions. Understanding the different models and country experiences is key to wider implementation. This event will feature pharmacy leadership bodies from across the world explaining their progress with access to records and how this impacts access to vaccination through pharmacies.
Watch video
Vaccines & special-risk groups: Pharmacy teams and all healthcare workers
March 9, 2023
Vaccination is one of the most effective measures to prevent disease transmission and protect populations from a variety of diseases that heavily impact individuals and health systems. Overall, through the provision of person-centred pharmaceutical services, pharmacists play a key role ensuring healthy lives and promoting wellbeing, as well as promoting more effective, rational, and widespread use of vaccines. Among the different special-risk population groups for vaccine-preventable diseases, healthcare professionals are in contact with multiple patients daily and are therefore at increased risk of contracting and further spreading vaccine-preventable diseases.
Watch video
Enabling life-course immunisation through pharmacy-based vaccination: Access to data and vaccination records
February 2, 2023
FIP is committed to supporting countries to develop policies that enable pharmacies and pharmacists to deliver integrated vaccination services throughout the life-course and as part of wider national immunisation policies. This event is delivered as part of a new FIP programme supported by Pfizer, which aims to provide our members with approaches and tools for tackling key policy enablers that determine equity, access and sustainability of pharmacy-based life-course immunisation.
The success of pharmacy-based vaccination depends on various policy factors, one of which is access to data and vaccination records. FIP data shows that pharmacists’ access to patient vaccination records vary widely across countries and regions. Understanding the different models and country experiences is key to wider implementation. This event will feature pharmacy leadership bodies from across the world explaining their progress with access to records and how this impacts access to vaccination through pharmacies.
Watch video
Enabling life-course immunisation through pharmacy-based vaccination: Regulations and prescribing
January 18, 2023
FIP is committed to supporting countries to develop policies that enable pharmacies and pharmacists to deliver integrated vaccination services throughout the life-course and as part of wider national immunisation policies. This event is delivered as part of a new FIP programme supported by Pfizer, which aims to provide our members with approaches and tools for tackling key policy enablers that determine equity, access and sustainability of pharmacy-based life-course immunisation.
Watch video
Enabling life-course immunisation through pharmacy-based vaccination: Service remuneration models
December 6, 2022
Remuneration of pharmacy vaccinations is one of the key policy enablers of pharmacy-based vaccinations, and thus life-course immunisation. FIP data shows that remuneration models vary widely across countries and regions. Understanding the different models and country experiences is key to wider implementation. This event will feature a number of pharmacy leadership bodies sharing their remuneration models and journeys for the benefit of policy-enablement everywhere.
This event is delivered as part of a new FIP programme supported by Pfizer, which aims to provide our members with approaches and tools for tackling key policy enablers that determine equity, access and sustainability of pharmacy-based life-course immunisation.
Watch video
Global symposium: Why flu vaccination matters
October 13, 2022
Despite a temporary drop in the incidence of seasonal influenza during the pandemic, it will continue to represent a heavy burden for healthcare systems and societies overall. In a post-pandemic scenario, it will be crucial to protect and further advance the progress that had been achieved globally in terms of flu vaccination rates. This event will discuss why flu vaccination remains a priority, and how co-administration of flu and COVID-19 vaccines can provide a convenient and effective solution for driving vaccination uptake for both diseases. The event will also explore how pharmacists can contribute to flu vaccination strategies.
Watch video
Co-administration of flu and COVID vaccines: Improving convenience and vaccination coverage
September 6, 2022
Influenza and COVID-19 can have an important impact in health, productivity and quality of life, especially for vulnerable populations. Likewise, both diseases have vaccines that are available and support a lower risk of transmission of the disease.
Pharmacists can play a major role in reminding people to get vaccinated, building vaccine confidence and supporting their decision making, dispensing vaccines and, in some countries, administering those vaccines. The coadministration of these vaccines can lead to an efficient and convenient way of improving vaccine uptake against both diseases. Community pharmacies’s accessibility and credibility put them in an ideal situation to provide this important service, thus making a valuable contribution to disease prevention.
Watch video
Le rôle des pharmaciens dans la prévention de la grippe et l’augmentation de la couverture vaccinale
September 1, 2022
This event will focus on pharmacists’ roles in influenza prevention through vaccination and increasing influenza vaccination coverage rate. The event will focus on the risks of influenza and showcase good practices from pharmacists across the continent on pharmacists’ public health role through influenza vaccination, digital health solutions in pharmacy on influenza vaccination and effective communication with patients to deliver flu vaccines on the right time. This event aims to improve pharmacists’ knowledge, improve pharmaceutical services delivery and overall health outcomes of the public in the context of influenza vaccination.
Watch video
Vaccines & special-risk groups: Cardiovascular diseases
July 21, 2022
Vaccination is one of the most effective measures to prevent disease transmission and protect populations from a variety of diseases that heavily impact individuals and health systems. Overall, through the provision of person-centred pharmaceutical services, pharmacists play a key role ensuring healthy lives and promoting wellbeing, as well as promoting more effective, rational, and widespread use of vaccines. Among the different special-risk population groups for vaccine-preventable diseases, people living with cardiovascular diseases have particular vaccination needs that will be highlighted at this event.
Learning objectives:
By the end of the session participants should be able to:
• Understand the risks that this particular population group is exposed to with regards to vaccine-preventable diseases, as well as the benefits of being vaccinated
• Understand the main recommended vaccines for people living with cardiovascular diseases
• Discuss the role of pharmacists in supporting health literacy and vaccination of this special risk group
Moderator:
– Renly Lim, FIP YPG Immediate Past President, Research Fellow University of South Australia, Australia
Panellists:
– Alvaro Sosa Liprandi, President Interamerican Society of Cardiology, Argentina
– Tolulope Osigbesan, Senior Policy and Advocacy Officer NCD Alliance, Switzerland
– John D. Grabenstein, RPh, PhD, Director of Scientific Communications Immunize.org, USA
Watch video
Vaccines & special-risk groups: Cardiovascular diseases (French translation)
July 21, 2022
Vaccination is one of the most effective measures to prevent disease transmission and protect populations from a variety of diseases that heavily impact individuals and health systems. Overall, through the provision of person-centred pharmaceutical services, pharmacists play a key role ensuring healthy lives and promoting wellbeing, as well as promoting more effective, rational, and widespread use of vaccines. Among the different special-risk population groups for vaccine-preventable diseases, people living with cardiovascular diseases have particular vaccination needs that will be highlighted at this event.
Learning objectives:
By the end of the session participants should be able to:
• Understand the risks that this particular population group is exposed to with regards to vaccine-preventable diseases, as well as the benefits of being vaccinated
• Understand the main recommended vaccines for people living with cardiovascular diseases
• Discuss the role of pharmacists in supporting health literacy and vaccination of this special risk group
Moderator:
– Renly Lim, FIP YPG Immediate Past President, Research Fellow University of South Australia, Australia
Panellists:
– Alvaro Sosa Liprandi, President Interamerican Society of Cardiology, Argentina
– Tolulope Osigbesan, Senior Policy and Advocacy Officer NCD Alliance, Switzerland
– John D. Grabenstein, RPh, PhD, Director of Scientific Communications Immunize.org, USA
Watch video
Vaccines & special-risk groups: Chronic Respiratory Conditions
June 29, 2022
Vaccination is one of the most effective measures to prevent disease transmission and protect populations from a variety of diseases that heavily impact individuals and health systems. Overall, through the provision of person-centred pharmaceutical services, pharmacists play a key role ensuring healthy lives and promoting wellbeing, as well as promoting more effective, rational, and widespread use of vaccines. Among the different special-risk population groups, people living with chronic respiratory conditions have specific vaccination needs that will be discussed in this event.
By the end of the session participants should be able to:
-Understand the risks that this particular population group is exposed to with regards to vaccine-preventable diseases, as well as the benefits of being vaccinated
-Understand the main recommended vaccines for people living with chronic respiratory diseases
-Discuss the role of pharmacists in supporting health literacy and vaccination of this special risk group
Moderator:
-Peter Guthrey, Executive Committee Member of the Social and Administrative Pharmacy Section of FIP; Senior pharmacists – strategic policy The Pharmaceutical Society of Australia - Australia
Panelists:
-Ema Paulino, President National Association of Pharmacies, Portugal (ANF), Portugal
-Fiona Mosgrove, General Practitioner NHS Grampian, UK
-Carmen Baldonedo Mosteiro, Community Pharmacist. Member of the Respiratory task force of the Spanish Society of community Pharmacy (SEFAC), Spanish society of clinical, familiar and community Pharmacy (SEFAC), Spain
Watch video
Vaccines & special-risk groups: Chronic Respiratory Conditions (French translation)
June 29, 2022
La vaccination est l’une des mesures les plus efficaces pour prévenir la transmission des maladies et protéger les populations contre une variété de maladies qui ont un impact important sur les individus et les systèmes de santé. Globalement, en fournissant des services pharmaceutiques centrés sur la personne, les pharmaciens jouent un rôle clé pour garantir une vie saine et promouvoir le bien-être, ainsi que pour favoriser une utilisation plus efficace, rationnelle et généralisée des vaccins. Parmi les différents groupes de population à risque, les personnes vivant avec une affection respiratoire chronique ont des besoins spécifiques en matière de vaccination qui seront discutés lors de cet événement.
À la fin de la session, les participants devraient être en mesure de :
-Comprendre les risques auxquels ce groupe de population particulier est exposé en ce qui concerne les maladies évitables par la vaccination, ainsi que les avantages d’être vacciné.
-Comprendre les principaux vaccins recommandés pour les personnes vivant avec des maladies respiratoires chroniques.
-Discuter du rôle des pharmaciens dans l’éducation à la santé et la vaccination de ce groupe à risque particulier.
Modérateur :
-Peter Guthrey, membre du comité exécutif de la section de la pharmacie sociale et administrative de la FIP ; pharmaciens seniors – politique stratégique The Pharmaceutical Society of Australia – Australie
Panélistes :
-Ema Paulino, Président de l’Association Nationale des Pharmacies, Portugal (ANF), Portugal
Fiona Mosgrove, médecin généraliste NHS Grampian, Royaume-Uni.
-Carmen Baldonedo Mosteiro, pharmacien communautaire. Membre du groupe de travail sur les maladies respiratoires de la Société espagnole de pharmacie communautaire (SEFAC), Société espagnole de pharmacie clinique, familiale et communautaire (SEFAC), Espagne.
Watch video
The role of community pharmacies in COVID-19 booster vaccination: Opportunities and supply chain challenges in a post-pandemic scenario
April 26, 2022
Around the world, community pharmacies play an increasingly consolidated role in vaccination. During the pandemic, pharmacies’ accessibility made them a convenient place for vaccination against COVID-19 in several countries, increasing vaccination coverage and helping achieve herd immunity. As the world slowly begins to emerge from the pandemic, this event will discuss the role of community pharmacies in COVID-19 booster vaccination, and their challenges in vaccine supply logistics and cold storage solutions, particularly in a post-pandemic scenario. Key interventions by community pharmacists in vaccine supply logistics linked to COVID-19 from different parts of the world will be presented.
Learning objectives:
1. Understand the supply chain and distribution process of COVID-19 vaccines from manufacturer to hospitals, community pharmacies, and the public;
2. Explore the role of community pharmacies in vaccine supply logistics for COVID-19;
3. Discuss the challenges of COVID-19 booster vaccination supply logistics and cold storage solutions in community pharmacies, particularly in a post-pandemic scenario;
4. Explore supply models that involve community pharmacies contributing to population vaccination coverage in a post-pandemic scenario.
Moderator:
– Catherine Duggan, Chief Executive Officer FIP, the Netherlands
Panellists:
– Vibhu Paudyal, Associate Professor in Clinical Pharmacy University of Birmingham, UK
– Ronald T. Piervincenzi, Ph.D., Chief Executive Officer USP, USA
– Loganathan Fahrni, Senior lecturer and pharmacist Universiti Teknologi MARA, Malaysia
Watch video
Advocating for the role of pharmacists in diphtheria-tetanus-pertussis booster, COVID-19, and meningococcal meningitis vaccinations
April 25, 2022
The importance of vaccines creates opportunities for pharmacists and pharmacies to contribute to improving vaccination coverage. Since the publication of FIP’s 2020 report on vaccinations highlighting the role of pharmacists and pharmacies in this area, there has been a significant expansion in pharmacy-based vaccination and the scope of vaccines delivered by pharmacists and the pharmacy workforce. To kick off World Immunisation Week, this digital event will provide an overview of that expansion for tetanus, diphtheria, acellular pertussis booster, COVID-19, and meningitis vaccines. Moreover, it will allow participants to hear from countries in various stages of advocating for a broader role for pharmacists in vaccination.
Watch video
Enabling multiprofessional vaccine prescribing and administration for improved uptake rates: Eastern Mediterranean Region
March 14, 2022
In many countries, vaccination coverage rates are suboptimal, and vaccination services are largely focused on childhood. Goals to expand vaccination pathways include to have more and more easily accessible vaccination points, and more professionals who can deliver the service to more people and offer evidence-based advice on vaccines. This event will discuss the drivers and barriers to interprofessional understanding, cooperation and task sharing in the area of vaccination in each region, and the regulatory authority granted to different healthcare professionals to prescribe and/or administer vaccines.
Watch video
Enabling multiprofessional vaccine prescribing and administration for improved uptake rates: Southeast Asian Region
March 10, 2022
In many countries, vaccination coverage rates are suboptimal, and vaccination services are largely focused on childhood. Goals to expand vaccination pathways include to have more and more easily accessible vaccination points, more professionals who can deliver the service to more people and offer evidence-based advice on vaccines. This event will discuss the drivers and barriers to interprofessional understanding, cooperation and task sharing in the area of vaccination in the region, and the regulatory authority granted to different healthcare professionals to prescribe and/or administer vaccines.
Watch video
Enabling multiprofessional vaccine prescribing and administration for improved uptake rates: African Region
March 7, 2022
In many countries, vaccination coverage rates are suboptimal, and vaccination services are largely focused on childhood. Goals to expand vaccination pathways include to have more and more easily accessible vaccination points, more professionals who can deliver the service to more people and offer evidence-based advice on vaccines. This event will discuss the drivers and barriers to interprofessional understanding, cooperation and task sharing in the area of vaccination in the region, and the regulatory authority granted to different healthcare professionals to prescribe and/or administer vaccines.
Watch video
Permettre la prescription et l’administration multiprofessionnelles des vaccins pour améliorer les taux de prise en charge: Région africaine
March 7, 2022
Dans de nombreux pays, les taux de couverture vaccinale sont sous-optimaux et les services de vaccination sont essentiellement axés sur l’enfance.
Les objectifs visant à développer les parcours de vaccination consistent notamment à disposer de points de vaccination plus nombreux et facilement accessibles, d’un plus grand nombre de professionnels capables de fournir le service à un plus grand nombre de personnes et d’offrir des conseils sur les vaccins fondés sur des données probantes.
Watch video
Enabling multiprofessional vaccine prescribing and administration for improved uptake rates: European Region
March 3, 2022
In many countries, vaccination coverage rates are suboptimal, and vaccination services are largely focused on childhood. Goals to expand vaccination pathways include to have more and more easily accessible vaccination points, and more professionals who can deliver the service to more people and offer evidence-based advice on vaccines. This event will discuss the drivers and barriers to interprofessional understanding, cooperation and task sharing in the area of vaccination in each region, and the regulatory authority granted to different healthcare professionals to prescribe and/or administer vaccines.
Watch video
Enabling multiprofessional vaccine prescribing and administration for improved uptake rates: Americas Region
March 1, 2022
In many countries, vaccination coverage rates are suboptimal, and vaccination services are largely focused on childhood. Goals to expand vaccination pathways include to have more and more easily accessible vaccination points, and more professionals who can deliver the service to more people and offer evidence-based advice on vaccines. This event will discuss the drivers and barriers to interprofessional understanding, cooperation and task sharing in the area of vaccination in each region, and the regulatory authority granted to different healthcare professionals to prescribe and/or administer vaccines.
Watch video
Permitir la prescripción y administración multiprofesional de vacunas para mejorar las tasas de vacunación: Región de las Américas
March 1, 2022
En muchos países, las tasas de cobertura de vacunación no son óptimas, y los servicios de vacunación se centran principalmente en la infancia. Los objetivos para ampliar las vías de acceso a la vacunación incluyen disponer de más puntos de vacunación de fácil acceso, más profesionales que puedan prestar el servicio a un mayor número de personas y ofrecer asesoramiento basado en la evidencia sobre las vacunas. En este evento se debatirán los factores que impulsan y obstaculizan el entendimiento interprofesional, la cooperación y el reparto de tareas en el ámbito de la vacunación en la región, así como la autoridad normativa concedida a los distintos profesionales sanitarios para prescribir y/o administrar vacunas.
Watch video
Enabling multiprofessional vaccine prescribing and administration for improved uptake rates: Western Pacific Region
February 28, 2022
In many countries, vaccination coverage rates are suboptimal, and vaccination services are largely focused on childhood. Goals to expand vaccination pathways include to have more and more easily accessible vaccination points, and more professionals who can deliver the service to more people and offer evidence-based advice on vaccines. This event will discuss the drivers and barriers to interprofessional understanding, cooperation and task sharing in the area of vaccination in each region, and the regulatory authority granted to different healthcare professionals to prescribe and/or administer vaccines.
Watch video
Leadership summit on pharmacy-based vaccination policy and advocacy
November 18, 2021
The Leadership Summit– the programme finale – is a high-level event in which we recap the primary outcomes of the 12-event digital series. Together, we will adopt the FIP Commitment to Action on sustainable and equitable access to vaccines through pharmacy which will be launched during the Summit. Global leaders from health and pharmacy join this landmark event.
Watch video
Presentación del documento técnico Servicios farmacéuticos en inmunización: aportes, experiencias e implementación en la región de las Américas.
November 17, 2021
El documento técnico, Servicios farmacéuticos en inmunización: Aportes, experiencias e implementación en la región de las Américas, elaborado por el Centro de Información de Medicamentos del Instituto de Investigaciones Farmacéuticas de la Facultad de Farmacia de la Universidad de Costa Rica para el Foro Farmacéutico de las Américas (FFA), ofrece una visión general de la participación del profesional en farmacia en temas de vacunas y en servicios farmacéuticos en inmunización en América Latina. En este webinar de presentación, se hará una reseña de sus contenidos y la relación con los objetivos de desarrollo de la Federación Internacional Farmacéutica (FIP). Este documento tiene la pretensión de constituirse en una herramienta para la implementación de servicios farmacéuticos en inmunización en la región que complementa las formuladas y emitidas por la FIP.
Watch video
Sustainable and equitable access to vaccines: Establishing priorities and setting policies in the African region
November 4, 2021
In the final sixth of six regions we dissect, event 11 focuses on the regional needs and drivers for transforming vaccination in the African region. This involves identifying priorities in the region’s countries with regards equity and access to vaccinations. We also discuss barriers and challenges and regional needs in relation to policy setting. FIP members, stakeholders and partners from the African region join us for a true regional perspective.
Watch video
Sustainable and equitable access to vaccines: Establishing priorities and setting policies in the Western Pacific region
October 28, 2021
In the fifth of six regions we dissect, event 10 focuses on the regional needs and drivers for transforming vaccination in the Western Pacific region. This involves identifying priorities in the region’s countries with regards equity and access to vaccinations. We also discuss barriers and challenges and regional needs in relation to policy setting. FIP members, stakeholders and partners from the Western Pacific region join us for a true regional perspective.
Watch video
Sustainable and equitable access to vaccines: Establishing priorities and setting policies in the South East Asian region
October 26, 2021
In the fourth of six regions we dissect, event 9 focuses on the regional needs and drivers for transforming vaccination in the South East Asian region. This involves identifying priorities in the region’s countries with regards equity and access to vaccinations. We also discuss barriers and challenges and regional needs in relation to policy setting. FIP members, stakeholders and partners from the South East Asian region join us for a true regional perspective.
Watch video
Joining forces across civil society organisations towards improved vaccination coverage
October 21, 2021
Vaccination coverage rates for several diseases are suboptimal and remain below WHO targets in many countries of all income levels. For FIP, it is essential to engage with civil society organisations representing different constituencies – from patient advocacy groups to older adult organisations and to vaccination advocates – to discuss and outline synergistic and complementary advocacy actions for broader access and convenience of vaccination services through a diversity of providers and pathways. This event gathered different civil society organisations to express their concerns, best practices and expectations, namely with regards to the role of pharmacists, for improving vaccination coverage, and to identify potential alignments in advocacy strategies.
Watch video
Sustainable and equitable access to vaccines: Establishing priorities and setting policies in the European region
October 14, 2021
In the third of six regions we focuses on the regional needs and drivers for transforming vaccination in the European region. This involves identifying priorities in the region’s countries with regards equity and access to vaccinations. We also discuss barriers and challenges and regional needs in relation to policy setting. FIP members, stakeholders and partners from the European region join us for a true regional perspective.
Watch video
Confidence, complacency and convenience: Key elements of influenza vaccination strategies in times of COVID-19
October 12, 2021
This digital event discussed how important it is to promote confidence in influenza vaccines and avoid complacency towards vaccination due to lower influenza rates in the past year. Harnessing the convenience of access to pharmacies will be paramount to achieve these goals. Due to the widespread adoption of disease prevention measures to reduce transmission of COVID-19, the prevalence of the seasonal influenza lowered drastically around the world. However, as more people become vaccinated against COVID-19 and measures such as mask wearing, social distancing and home-based working or schooling are eased, the flu could return with high incidence rates and people could be more susceptible to the influenza virus due to lack of recent exposure. Developing flu vaccines this year has also been more challenging than usual due to less data available. This uncertainty around the return of influenza makes vaccination all the more critical, and it essential to communicate this to the population.
Watch video
Sustainable and equitable access to vaccines: Establishing priorities and setting policies in the Eastern Mediterranean region
October 7, 2021
In the second of six regions we dissect, event 7 focuses on the regional needs and drivers for transforming vaccination in the Eastern Mediterranean region. This involves identifying priorities in the region’s countries with regards equity and access to vaccinations. We also discuss barriers and challenges and regional needs in relation to policy setting. FIP members, stakeholders and partners from the Eastern Mediterranean region join us for a true regional perspective.
Watch video
Acceso sostenible y equitativo a las vacunas: Establecimiento de prioridades y fijación de políticas en la región de las Américas
October 1, 2021
En la primera de las seis regiones que analizaremos, este evento se centrará en las necesidades y los catalizadores a nivel regional para transformar las estrategias de vacunación en las Américas. Se trata de identificar las prioridades de los países de la región en materia de equidad y acceso a la vacunación. También discutiremos las barreras, los desafíos y las necesidades regionales en relación con el establecimiento de políticas de vacunación. Los miembros de la FIP, y otras organizaciones y socios relevantes de la región de las Américas participarán conjuntamente para obtener una verdadera perspectiva regional sobre este asunto.
Watch video
Sustainable and equitable access to vaccines: Establishing priorities and setting policies in the Americas region
September 30, 2021
In the first of six regions we dissect, event 6 focuses on the regional needs and drivers for transforming vaccination in the Americas. This involves identifying priorities in the region’s countries with regards equity and access to vaccinations. We also discuss barriers and challenges and regional needs in relation to policy setting. FIP members, stakeholders and partners from the Americas region, join us for a true regional perspective.
Watch video
Working together across systems to transform vaccination policy: working with others in our professions, with other disciplines and agencies to establish sustainable policies
September 23, 2021
This event focused on intra and interprofessional alliances to ensure that we can work together to deliver the areas of policy addressed in this series. Panellists discussed working together with other agencies and policymakers to establish sustainable policies on vaccines access and equity, including how pharmacy can be a force of good in supporting vaccinations for all health workers.
Watch video
Understanding vaccine hesitancy and building vaccine confidence through effective conversations
September 8, 2021
The World Health Organization has listed vaccine hesitancy — concerns related to vaccination or outright refusal to receive vaccines despite availability — as one of the top 10 threats to global health in 2019. This threat is evidenced by recent outbreaks of vaccine-preventable diseases in many high-income countries and the sheer volume of disinformation circulating regarding the recently approved vaccines against COVID-19.
Watch video
Health illiteracy and vaccine misinformation as determinants for equity: developing policies to establish access to quality information in an equitable way
August 26, 2021
This event focused on the issues around health literacy and access to information. The societal influences on the ‘’infodemic’’ will be laid out, also the role of health care professionals, health workers and be vaccine hesitant people. We considered all the policy elements that need to be addressed to overcome this, identifying tools to overcome these issues from a range of angles and the role pharmacy can play.
Watch video
Vaccinations and the genders: Examining inequities in gender access and handling of vaccinations globally to inform pharmacy policy
August 10, 2021
Episode 3 examines inequities in gender access to vaccines to inform pharmacy policy. Various angles to vaccinations and the gender will be discussed including general gender access, terms of empowering women as caregivers to support vaccinations, the impact of COVID-19 on gender inequity, and the issues around gender inequity in the workforce (which is especially important to discuss in 2020 Year of the Health Worker).
Watch Video
Developing Effective Pharmacy-led Vaccination Campaigns
July 26, 2021
The World Health Organization lists vaccine hesitancy—concerns related to vaccination or outright refusal to receive vaccines despite availability—as one of the top ten threats to global health in 2019. This threat is evidenced by recent outbreaks of vaccine preventable diseases in many high-income countries and the sheer volume of disinformation circulating regarding the recently approved vaccines against novel Sars-Cov-2 virus.
Often times, “one-size-fits-all” vaccine promotion efforts can backfire when presented to individuals who are already sceptical of vaccines. This event will outline evidenced-based strategies for developing pharmacy-led immunization campaigns which communicate the value of vaccines to specific patient groups in order to build vaccine confidence and improve uptake.
Watch Video
Equity, access & sustainability through life’s ages and stages: Enabling a life course-approach to vaccination
July 8, 2021
Episode 2 takes a life-course approach to discussing vaccination. The panelists will focus on age and explore vaccines equity and access through life’s ages and stages – from childhood (including pregnancy) to later adulthood – from a pharmacy perspective. They will focus on setting out the policy issues and immediate next steps, determining what pharmacy needs to do to singularly affect the most change in this area.
Watch Video
Joining forces across health professionals towards improved vaccination coverage
June 29, 2021
Vaccination coverage rates for several diseases are suboptimal and remain below WHO targets in many countries of all income levels. As such, FIP considers that it is essential to promote dialogue and understanding around these perceptions among healthcare professionals’ organisations at the international level, so that these can be cascaded to the regional, country and local levels.
Watch Video
Introducing the FIP ‘Transforming Vaccination Globally, Regional and Nationally’ 2021: Accelerating equity, access and sustainability through policy development and implementation
June 17, 2021
Episode 1, the digital programme’s Opening Event, will set the stage for the entire programme. Together we will revisit the key outcomes of the Transforming Vaccination 2020 programme, specifically focusing on the outcomes relating to shaping policy around equity, access and sustainability and how they are shaping the FIP vaccination agenda for 2021. We will also describe this year’s programme, outline its key aims and objectives as well as outcomes. This special programme opener is for anyone invested in transforming and shaping vaccination in pharmacy!
Watch Video
Pharmacists’ involvement in COVID-19 vaccination: Addressing regulatory needs
February 4, 2021
FIP’s Regulators’ Forum presented a new regulatory vaccination preparedness self-assessment tool and risk assessment. Foundational regulatory needs were presenteded to ensure oversight, quality assurance and public protection as the scope of pharmacist’s patient care is expanded to include prescribing, vaccination and testing in order to address population health needs during a pandemic. A brief overview of the data collected by FIP on the role of pharmacists in vaccinations and other FIP documents was also presented.
Watch Video
Global Immunization Initiatives: Implementing Lifelong Learning to Address Mass Vaccination
January 21, 2021
This session will examine the application of the concepts of the Health Belief Model (HBM) which is one of the first and still most commonly used frameworks or theories of health behavior. There has been successful application of the HBM in health and wellness programs, including immunization and chronic diseases management. As such, the application of HBM will be illustrated in a flu awareness campaign that targeted Lebanese University students. Examples of improving immunization rates and addressing patient perceptions in an ambulatory care setting in the U.S. will also be discussed.
Watch Video
Vaccine administration routes and procedures: frequent concerns and common errors
January 13, 2021
Although pharmacists have administered vaccines in several countries for years, this role is still new or even unknown to the profession in many parts of the world. As a role that involves direct contact with the patient and the administration of a product by injection, it may still generate some concerns among pharmacists with regards to the administration procedure, common errors and the management of anaphylactic reactions. This webinar will describe such procedures and address common questions and concerns.
Watch Video
FIP Global Virtual Summit: Committing to action to transform vaccination in pharmacy
December 17, 2020
This global virtual Summit – the programme finale – is a high-level event in which we will recap the primary outcomes of the 24-event digital series. Together, we will adopt the FIP Commitment to Action on Vaccination in Pharmacy which will be launched during the Summit. Global leaders from health and pharmacy join this landmark event.
Watch Video
From regional to global: Common priorities for action to transform vaccination
December 15, 2020
This roundtable event follows 6 regional events – episodes 3.1 to 3.6 of the programme – which dissected priorties, needs and barriers across the world’s six regions. We now bring back the key outcomes from the six regional discussions int one roundtable to identify common priorities for action to transform vaccination. Each regional perspective will be represented by the panelists, alongside other leaders and stakeholders.
Watch Video
Necesidades e impulsores regionales de la transformación de la vacunación: Américas
December 10, 2020
Event 3.6 focuses on the regional needs and drivers for transforming vaccination in the Americas region. This involves discussing the progress made in countries in the Americas with regards to pharmacist vaccination, the quality and universal access to vaccinations. We also discuss barriers and challenges and regional needs for future transformation.
Watch Video
Regional needs and drivers for transforming vaccination: Africa
December 9, 2020
In the fifth of six regions we dissect, event 3.5 focuses on the regional needs and drivers for transforming vaccination in the African region. This involves discussing the progress made in Africa with regards to pharmacist vaccination, the quality and universal access to vaccinations. We also discuss barriers and challenges and regional needs for future transformation. FIP members, stakeholders and partners from Africa join us for a true regional perspective.
Watch Video
Regional needs and drivers for transforming vaccination: Western Pacific
December 8, 2020
In the fourth of six regions we dissect, event 3.4 focuses on the regional needs and drivers for transforming vaccination in the Western Pacific. This involves discussing the progress made in Western Pacific countries with regards to pharmacist vaccination, the quality and universal access to vaccinations. We also discuss barriers and challenges and regional needs for future transformation. FIP members, stakeholders and partners from the Western Pacific join us for a true regional perspective.
Watch Video
Regional needs and drivers for transforming vaccination: Eastern Mediterranean
December 3, 2020
In the third of six regions we dissect, event 3.3 focuses on the regional needs and drivers for transforming vaccination in the Eastern Mediterranean region (EMR). This involves discussing the progress made in EMR countries with regards to pharmacist vaccination, the quality and universal access to vaccinations. We also discuss barriers and challenges and regional needs for future transformation. FIP members, stakeholders and partners from the EMR join us for a true regional perspective.
Watch Video
Regional needs and drivers for transforming vaccination: South East Asia
December 2, 2020
In the second of six regions we dissect, event 3.2 focuses on the regional needs and drivers for transforming vaccination in the South East Asian region (SEAR). This involves discussing the progress made in SEAR countries with regards to pharmacist vaccination, the quality and universal access to vaccinations. We also discuss barriers and challenges and regional needs for future transformation. FIP members, stakeholders and partners from the SEAR join us for a true regional perspective.
Watch Video
Regional needs and drivers for transforming vaccination: Europe
December 1, 2020
In the first of six regions we dissect, event 3.1 focuses on the regional needs and drivers for transforming vaccination in Europe. This involves discussing the progress made in European countries with regards to pharmacist vaccination, the quality and universal access to vaccinations. We also discuss barriers and challenges and regional needs for future transformation. FIP members, stakeholders and partners from the European region join us for a true regional perspective.
Watch Video
Communicating vaccine safety, building vaccine confidence
November 27, 2020
This event will discuss how the quality, efficacy and safety of vaccines are built into their development and manufacturing processes, including the clinical research they undergo and the strict regulatory assessment that precedes their marketing authorization. In addition, speakers will discuss how to make such processes understood and well-known by the public in a transparent and clear manner, in order to build confidence in vaccines and address the grave problem of vaccine hesitancy and anti-vaccines movements, i.e., the refusal to get vaccinated despite the availability of vaccines.
Watch Video
Setting transformative goals for vaccination globally
November 25, 2020
In this episode – “Setting Transformation Goals for Vaccination Globally” – is a roundtable discussion that focuses on the role of the FIP Development Goals in the transformation of pharmacist vaccination globally and regionally. The discussion will focus on the development of transformative goals and how these goals will be used to deliver pharmacist vaccinators in all jurisdictions, resulting in increased vaccination rates worldwide
Watch Video
Pharmacist vaccinators and patient safety: FIP DG 19 Patient Safety
November 11, 2020
In this episode – “Pharmacist Vaccinators as a way to improve access to medicines” – is an interview format that focuses on the FIP Development Goal 18: Access to Medicines and Services from the perspective of vaccination by a pharmacist. The role of pharmacists in ensuring that vaccinations are widely and easily accessible by all people in all areas of the world, and the expertise of pharmacists in delivering the logistics and services needed to increase vaccination rates.
Watch Video
Pharmacist vaccinators and antimicrobial stewardship: FIP DG 17 Antimicrobial Stewardship
November 11, 2020
In this episode – “Pharmacist Vaccinators and Antimicrobial Stewardship” – is an interview format that focuses on the FIP Development Goal 17: Antimicrobial Stewardship from the perspective of vaccination by a pharmacist. The role of vaccination in antimicrobial stewardship, preserving antimicrobial effectiveness, preventing antimicrobial resistance, and the promise of addressing these issues that pharmacists vaccinators will deliver.
Watch Video
Pharmacist vaccinators and communicable disease management: FIP DG 16 Communicable Diseases
November 10, 2020
In this episode – “Pharmacist Vaccinators and Communicable Disease Management” – is an interview format that focuses on the FIP Development Goal 16: communicable and Vector Diseases from the perspective of vaccination by a pharmacist. The role of vaccination in the prevention and suppression of communicable and vector-borne disease, and the promise of addressing these diseases that pharmacists vaccinators will deliver.
Watch Video
Removing policy barriers to pharmacist vaccinations: FIP DG 13 Policy Development
November 5, 2020
In this episode, “Removing Policy Barriers to Pharmacist Vaccination” – is an interview format that focuses on the FIP Development Goal 13: Policy Development from the perspective of vaccination by a pharmacist. We discuss the process of policy development, identification of barriers, and engagement with stakeholders to ensure that pharmacist vaccination is accessible to all people in all settings where pharmacists work.
Watch Video
Empowering pharmacists to deliver vaccination at the health-system level: FIP DG 7 Advancing Integrated Services
November 3, 2020
In this episode, “Empowering Pharmacists to Deliver Vaccination in all Settings” – is an interview format that focuses on the FIP Development Goal 7: Advanced Integrated Services from the perspective of vaccination by pharmacist. We discuss change management principles in the context of integrating advanced practice services into every day practice, ensuring that vaccination by pharmacists in all settings and jurisdictions is consistent with best evidence and practice guidelines. Where a pharmacist is present in all settings, vaccinations can be provided. Ensuring collaborative work and shared documentation with all healthcare professionals to ensure it is an integrated service.
Watch Video
Vaccination from specialist practice to every day practice: FIP DG 4 Advanced & Specialist Development
October 28, 2020
In this episode – “Vaccination: From Specialist Practice to Everyday Practice” – is an interview format that focuses on the FIP Development Goal 4: Advanced & Specialist Development from the perspective of vaccination by a pharmacist. We discuss the process required to take vaccination by pharmacists from a specialized field that few pharmacists deliver in some jurisdictions, to a standard part of pharmacists’ care in all settings and locations.
Watch Video
L’hiver arrive : La vaccination contre la grippe en période de COVID-19. Bonnes pratiques des pays francophones
October 22, 2020
La grippe saisonnière représente une charge de morbidité importante pour le système de santé et un risque pour plusieurs groupes de population, notamment les personnes âgées, les personnes souffrant d’affections sous-jacentes, les femmes enceintes et d’autres.
La menace d’une deuxième épidémie de COVID-19 est très réelle, et le risque que les deux virus entraînent une morbidité et une mortalité combinées ne peut être négligé.
Watch Video
Reviewing the FIP Transforming Vaccination Programme: Enabling and supporting our profession
October 21, 2020
Scope: Transforming Vaccination with the FIP Development Goals: Brining practice, science and workforce together
What were the key insights and summaries from each of the events so far?
What was the one key message you gathered from that presentation, what was the main need identified from the presentation?
What is one key change you think that could be implemented to address this change?
Watch Video
FIP Transforming Vaccination Globally and Regionally. Transforming our workforce: Evolving the pharmacist’s qualification
Avances en el desarrollo de vacunas contra COVID-19 e importancia de la vacunación en tiempos de pandemia
October 7, 2020
Tras la irrupción de la infección respiratoria por el nuevo coronavirus SARS-CoV-2, el desarrollo de vacunas capaces de proporcionar protección individual y poblacional frente a este virus se presenta como uno de los pilares fundamentales para controlar su expansión. Por otro lado, entre los numerosos efectos de la pandemia actual, conviene destacar los relacionados con la interrupción de los servicios básicos de salud, entre ellos, los programas de vacunación que están sufriendo importantes caídas y demoras en la mayoría de los países. En esta conferencia se abordarán las diferentes vacunas que se están en progreso para COVID-19, las tecnologías utilizadas y los adelantos en su desarrollo, así como la importancia de reestablecer y mantener los sistemas de inmunización.
Watch Video
From smallpox to COVID-19: Vaccine development & innovation
Addressing barriers to uptake: Adherence, misinformation & anti-Science
Enabling practice: Empowering pharmacists & removing barriers
Transforming practice: A focus on strategy & policy for global change
Introducing the FIP ‘Transforming Vaccination Globally & Regionally’ series Programme… needs, action and outcomes
The value of vaccines for society and special populations
Influenza vaccination – Strategic elements of development, supply and delivery for optimal prevention
Can the world afford low vaccination coverage rates? Broadening vaccination gateways through pharmacies
Give it a shot: Advocating for pharmacy-based vaccination and achieving legislative changes
An overview of current pharmacy impact on immunisation – Presentation of key findings from FIP’s global report 2020
Increasing vaccination coverage through pharmacists | Episode 2: Who’s immune to fake news? Addressing patient motivation and vaccine hesitancy
Winter is coming: Influenza vaccination in times of COVID19–Best practices from Southern Hemisphere
Mass vaccination campaigns
Partnerships
Partnerships are crucial to advocate for a broader role for pharmacy in vaccination. Over the years, FIP has collaborated and joined efforts with several organisations with aligned agendas in the area of vaccination.
The different organisations that have worked with FIP in this area are highlighted in this section, including the details of their partnership and areas of collaboration.

Influenza Diabetes Community
Founded in 1998, The European Scientific Working group on Influenza and other Respiratory Viruses (ESWI) is a network organisation that aims to reduce the burden of influenza and other acute respiratory viruses in Europe. The Influenza Diabetes Community is a focus area for ESWI and serves as a virtual reference center, containing materials supplied by the IDC members on the importance of influenza vaccination for people with diabetes.
People with diabetes still face a higher risk for influenza and its complications. National and international guidelines therefore advise that diabetes patients be annually vaccinated against influenza. More information can be found in the Influenza Diabetes Community website.
FIP is a member of the IDC since 2020. This gives us the opportunity to voice and advocate for the role that pharmacists can play both in immunisation and in the care of people living with diabetes. It also gives us access to an interdisciplinary forum of experts in this area, which supports FIP in advancing our work on these topics.
IFAA – Immunisation for all ages
Initiative Overview
The goal of the Immunisation for All Ages initiative is to accelerate and advance policies that advocate for life-course vaccination by communicating the broader societal value of vaccination for all stages of life at a global and local level, such that policymakers, NGOs, governments, healthcare providers (HCPs) and citizens will support policies that prioritize vaccinations. The mission of IFAA is to expand and improve public awareness and policies that advocate for life course vaccination by learning from and building upon good practices and championing initiatives that will protect lives at all stages around the world. The IFAA is supported by Pfizer.
Steering Committee
The Immunisation for All Ages Steering Committee is responsible for guiding the direction and activities for the initiative with two overarching objectives:
- Identify where the Immunisation for All Ages network is uniquely positioned to address the current challenges, expand awareness, acceptance and access to life-course vaccination advocacy, as well as conduct strategic action planning using insights of the evolving global landscape for life course vaccination;
- Provide guidance and advice on leveraging annual summits to advance the collective strategic vision and lead initiatives that will be supported by a working group of other Immunisation for All Ages initiative participants or members.
Current Steering Committee members include:
- Dr. Jane Barratt, Global Advisor, International Federation of Ageing (IFA)
- Prof. Michael Moore AM PhD, Chair, Immunization Task Force, World Federation of Public Health Associations (WFPHA)
- Gonçalo Sousa Pinto, Lead for Practice Transformation, International Pharmaceutical Federation (FIP)
- David Sinclair, Chief Executive, International Longevity Centre (ILC-UK)
- Dr. Stefania Maggi, President, European Interdisciplinary Council on Ageing
- Prof. Charles Feldman, Professor of Pulmonology, University of Witwatersrand
Urgent work is needed to ensure any disruption to immunisation programmes from the pandemic is mitigated to protect the decades of progress made against vaccine-preventable diseases. Routine immunisation and efficient catch-up strategies for people at all stages of life are key elements needed for the health, well-being and prosperity of communities and countries. Given the disruptions inflicted by the pandemic on immunisation programmes, innovative solutions must be found to maximise routine immunisation uptake across all countries.
In addition to the well-known benefits of childhood immunisation, vaccines help to protect health throughout life and are a key component of healthy ageing. In particular, immunisation programmes for adult respiratory diseases such as influenza can substantially mitigate the annual burden of disease (increased mortality, morbidity, and healthcare costs), particularly among populations at greater risk of infection. Provision of vaccination by pharmacists is cost-effective and known to increase vaccination uptake. Increased utilisation of pharmacists will help alleviate pressure on current vaccine infrastructure, reduce potential disruption to routine immunisation beyond infancy, and strengthen health system capacity and vaccine coverage.
Immunisation plays a central role in the health promotion and prevention agenda and is a key component of healthy ageing and universal health coverage. Current health systems need to reorient from treating disease to preventing it at all stages of life. For example, investing in influenza vaccination saves time, money and lives, and leads to healthier, more sustainable healthcare systems and communities.
Learn through this webinar how global strategies such as the WHO Decade of Healthy Ageing (2020-2030) and WHO Immunisation Agenda 2030: A Global Strategy can be meaningfully implemented at a national level and what role civil society can play in driving change through the three interconnected pillars of prevention, access and equity.
Meningitis is deadly and debilitating. It strikes quickly, has serious health, economic and social consequences, and affects people of all ages, but particularly children under 5 years of age. If all people are to gain access to life-saving vaccines, major challenges must be overcome – vaccines must be delivered to marginalized populations, to displaced and migrating individuals, and to those affected by conflict, political instability and natural disasters. New approaches are needed to reach populations other than infants and to deliver integrated immunization and other health services in people-centred ways, as a part of primary health care to reach universal health coverage. This webinar explores the steps to implementing the roadmap for defeating meningitis, toward improving awareness and achieving higher vaccine coverage at a country level.

International Federation of Pharmaceutical Manufacturers and Associations – Influenza Vaccine Supply – IFPMA IVS
The Covid-19 pandemic has taught us a lot. Amongst other things, we’ve learnt about the importance of protecting the most vulnerable people in our societies, particularly older adults and patients with underlying medical conditions.
We need to improve influenza vaccination rates and, in doing so, reduce the burden of influenza disease nationally, regionally and globally. That’s why the IFPMA, IFA and FIP have joined forces to develop a common basis for a network communication strategy around influenza vaccination.
We’ve been working on our #TogetherAgainstFlu online campaign, together with the digital agency ZN. The objective is to keep raising awareness of the importance of flu vaccination and herd immunity for healthcare workers, older adults and patients living with chronic conditions, such as respiratory and heart diseases.
We are taking a network approach to reach these audiences. Evidence-based messages targeted at different stakeholders, opinion leaders and decision-makers will support their advocacy for implementation of existing recommendations for the prevention and control of seasonal influenza.
And we are looking to expand our influenza network. We are looking for other groups/organizations to partner with us in sharing the campaign messages across their audiences.
Join the influenza network. Help us spread us the messages around the importance of flu vaccination.
Vaccines Today
Vaccines Today has been online since 2011. The site is supported by Vaccines Europe, independently accredited by Health on the Net and is an active member of the WHO Vaccine Safety Net network. Its content is written by professional health journalists and actively reviewed by an Editorial Board of doctors, scientists and patient representatives. FIP is proudly joining this group of more than 20 organisations including the Immunization Action Coalition, History of Vaccines, the World Medical Association and Vaccine Ambassadors in the promotion of vaccination uptake and coverage around the world.
TBPPM Learning Network
The TBPPM Learning Network is a dynamic online community, fostering South-South learning and exchanges to engage private providers in TB prevention and care. With over 2600 members and organizations, the community convenes in webinars, discussion forums, workshops and documents lessons learned in Feature stories and knowledge products. The online platform functions as a resource center, learning stage and connector.
"Let's talk about vaccines!" Campaign
Pharmacists can play an important role in actively promoting vaccination, raising awareness and educating the population about the health, social and humanistic benefits of vaccines, including gains in quality of life. In particular, they can inform the community about such benefits through generic messaging (posters, videos, etc.) as well as targeted messages to individuals through conversations and advice.
Vulnerable populations like older adults, those living with chronic non-communicable diseases, or pregnant individuals are more likely to be severely impacted by vaccine-preventable diseases and therefore need to make informed decisions about getting vaccinated. For that, they need reliable, accurate and understandable information on vaccines from reputable sources and healthcare professionals, including pharmacists.
By developing a set of materials for pharmacy teams and for patients and the community, and a broad campaign about the benefits of vaccination for the different population groups, FIP aims to support its member organisations and also individual pharmacists in integrating such messages and conversations with patients into their daily practice, and support them in building confidence in vaccines and increasing vaccine uptake.
This campaign is supported through an unrestricted grant from Sanofi.


Resources for pharmacy professionals
In this section, pharmacy professionals can find a set of tools and resources that they can use to support their own professional development and in initiating conversations with patients and the community about vaccination.
These include videos, guidance and infographics. We invite you to visit this webpage regularly to check for new materials and translations.
CPD videos
These CPD Bites are short videos for pharmacists on specific vaccines and their use in risk population groups.
Episode 1: Pharmacists' roles in vaccination

Episode 2: Benefits of influenza vaccination in special populations

Episode 3: Benefits of pertussis vaccination in special populations

CPD Bites
These CPD Bites are short briefings for pharmacists on specific vaccines and their use in risk population groups.
Pharmacists' role in vaccination

Understanding influenza

Understanding pertussis

Infographic
This infographic is a summary of the main recommended vaccines and the risk groups that benefit from them.
Summary infographic of vaccination for risk groups
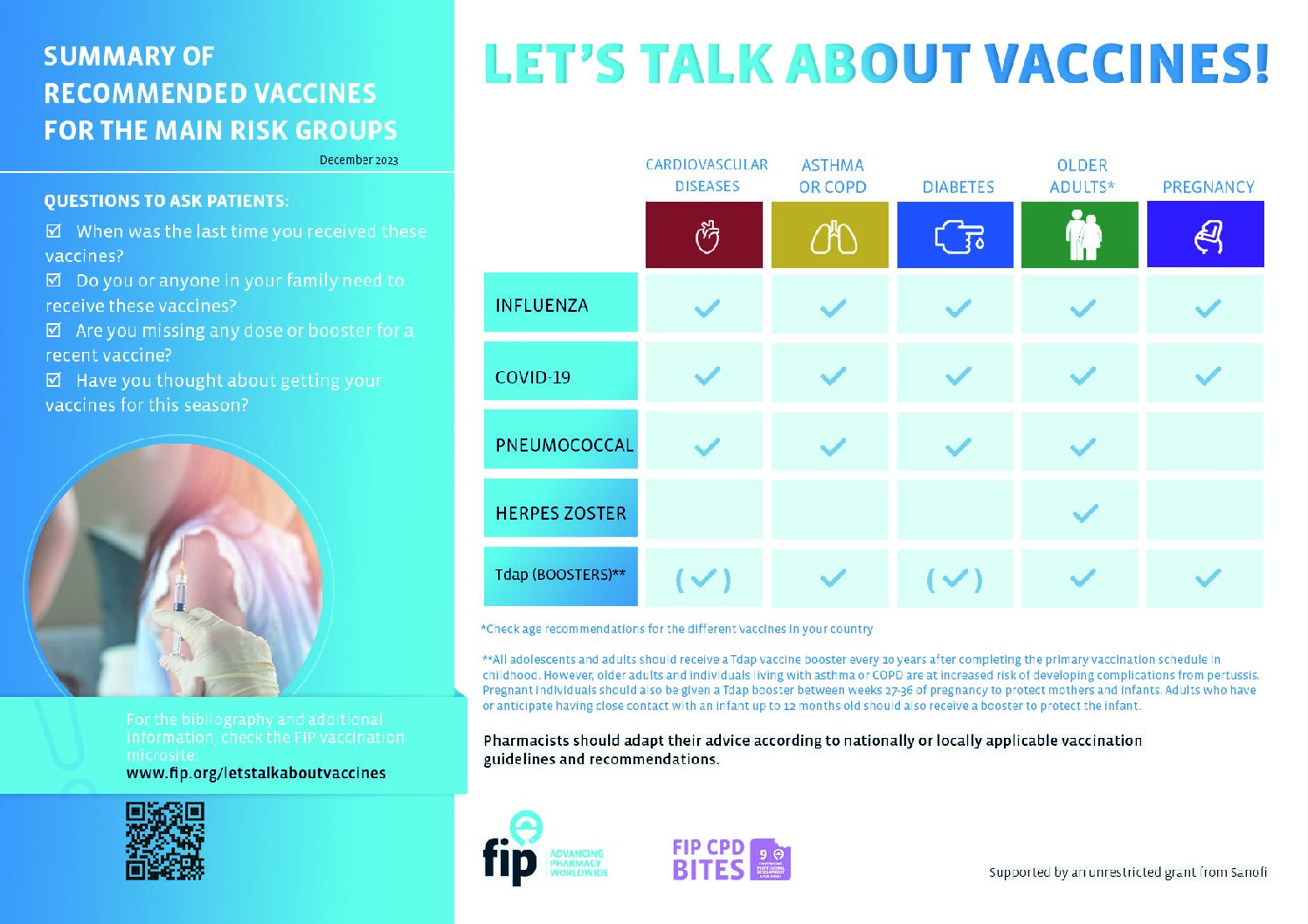
Resources for patients or the general public
Vulnerable populations, such as individuals with chronic conditions, older adults and pregnant people need reliable, accurate, and understandable information about vaccines from trusted sources. In this section, FIP provides a range of resources to be displayed at pharmacies or offered to patients and community members, to help them make informed decisions about getting vaccinated, protecting their health, and safeguarding the well-being of the community.
The materials in this page will help understanding how vaccines contribute to an improved quality of life and support learning about the importance of vaccination for different life stages and health conditions.
Video for pharmacy screens
This video can be displayed at the pharmacy. It presents the campaign resources.
Video presentation for pharmacy screens

Vaccination needs self-check
This poster can be printed and displayed at the pharmacy. It prompts the public to ask themselves a series of questions about their vaccination status and needs and invites them to ask their pharmacist for advice.
Vaccination needs self-check tool

Bibliography
This bibliography includes the references used to support this campaign and cited in the various materials.
Bibliography

Colophon
Let’s talk about vaccines!” campaign
Copyright 2023 International Pharmaceutical Federation (FIP)
Authors:
Gonçalo Sousa Pinto, FIP Lead for Practice Development and Transformation
Dr Dalia Bajis, FIP Lead for Partnerships and Provision
Dr Ozge Ozer, FIP Education and Professional Development Manager
Rúben Viegas, FIP Practice Development and Transformation Projects Coordinator
Design and layout:
Daria van Beek, FIP Marketing Manager
Hala Fathima, FIP Marketing Coordinator
Acknowledgments:
Amira Mustafa, FIP remote intern, USA (Content development)
Dr. Eric J. Yager, Albany College of Pharmacy and Health Sciences, USA (Reviewer)
Dr. Mine Durusu Tanriover, Hacettepe University, Turkiye (Reviewer)
CPD Bites/Videos
Addressing Vaccine Fatigue, Complacency and Confidence (2024)
Episode 1- What can pharmacists in low- and middle-income countries do about adult vaccine mistrust?
In this first episode, Dr Kate O’Brien, director of the World Health Organization’s Department of Immunization, Vaccines and Biologicals, explores the strategies that pharmacists can employ to address vaccine mistrust, particularly in low- and middle-income countries.
Watch here

Episode 2- What is vaccine complacency and fatigue and how can pharmacists address them among at-risk adults?
In this second episode, Dr Samantha Marsh, a Senior research fellow at the Department of General Practice and Primary Healthcare, University of Auckland, shares insights on how pharmacists can identify and address flu vaccine complacency and fatigue among at-risk adults.
Watch here

Episode 3- Using behavioural science to address vaccine concerns and hesitancy: how can pharmacists build confidence in adult vaccination, and particularly flu vaccination?
In this last episode, Gonçalo Sousa Pinto, FIP lead for Practice Development and Transformation, shares practical approaches that pharmacists can employ to tack vaccine hesitancy, and improve patients’ confidence in flu vaccination.
Watch here